Original Article
Epstein-.Barr virus-infected lymphocVtes in non-neoplastic lymph nodes of patients infected with human immunodeficiency virus
Heng Zheng and Shigeo Mori
Department of Pathology, The Institute of Medical Science, The University of Tokyo, Japan
Non-Hodgkin'S lymphomas occurring in AIDS patients (AIDS-NHL) are known to be highly associated with Epstein-Barr virus (EBV). However, there have been no previous detailed studies of the distribution of EBV-infected non-neoplastic lymphoblasts, which may act as precursors of AIDS-NHL. ln the present study, an attempt was made to detect such EBV-infected cells in patients' lymphoid organs. Fifteen non-neoplastic lymph nodes obtained from HIV-positive individuals were processed for in situ demonstration of EBV-encoded small RNA (EBER-1) and EBV-encoded latent membrane protein 1 (LMP-1). An increased number of EBER-1-expressing cells were observed (9/15). EBER-1-positive cells were present much more frequently in advanced cases,as evaluated using the histopathologic criteria of Grund-mann (1/6 cases showing irregular follicular hyperplasia; 3/4 showing the beginning follicular destruction; 2/2 showing progressive follicular destruction; 3/3 showing follicular involution). LMP-1 was detected in 3/9 EBER-1 -positive cases,and all three of these cases were at the most advanced stage. Furthermore, cells expressing LMP-1 were larger (62.83±6μm2) than EBER-1 + LMP-1- cells (29.05±7μm2).These results indicate that cells with latent expression of the EBV gene increase in number in lymphoid organs of HIV-infected individuals at an advanced stage and that some of the cells are in a transformed state. It is possible to speculate that these cells are precursors of AIDS-NHL.
Key words: Epstein-Barr, Epstein-Barr virus-encoded RNA,latent membrane protein, lymphoma
Around one-third of Japanese patients with acquired immunodeficiency syndrome (AIDS) have non-Hodgkin's malignant lymphoma (AIDS-NHL) as a complication.1-3 Epstein-Barr virus (EBV) has been suggested to play a causative role in most of these cases.4,5 EBV is a welI-known human oncogenic virus. Upon transformation, which is induced by chance through activation of EBV-infected host cells, EBV-encoded latent gene products such as EBV nuclear antigens l and 2 (EBNA-l, 2), latent membrane protein 1 (LMP-l) and EBV-encoded small RNA-l (EBER-l) are expressed.6 Such EBNA and LMP are antigenic to the host and thus can easily be targeted by killer cells if the host retains a normal immunological state. However, in immunocompromised states like acquired immune deficiency syndrome (AIDS), transformed B lymphoblasts can escape from immunologic surveillance, and thus can survive and proliferate in the host to form malignant tumors. This view, however, is merely hypothetical, and such EBV-transformed lymphocytes in various organs of immunocompromised patients have never been identified. In fact, only a very small number of previous reports have referred to EBV-infected lymphocytes in AIDS patients. Therefore, the present study was conducted to identify EBV-transformed lymphocytes in HIV-infected lymph nodes of patients without AIDS-NHL. First, we identified EBER-1-expressing cells using in situ hybridization (ISH). EBER-1 is a small RNA that is expressed in latent EBV-infected cells. It exists abundantly in the nuclei as ribonucleoprotejn, and is currently regarded as one of the best indices for determining EBV infection of lymphocytes. Second, we examined whether another EBV-encoded oncogenic gene product, LMP-1, is expressed in EBER-l-expressing cells. LMP-l is an EBV-encoded membrane protein that is known to be capable of transforming host cells.7 Finally, we measured the size of EBER-1 + LMP-1 + cells to clarify whether the double-positive cells are in a state of blastic transformation.
Correspondence: Heng Zheng, MD, Department of Pathology, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108, Japan.
Received 30 July l996. Accepted for publication 28 October 1996.
MATERIALS AND METHODS
Materials
Formalin-fixed, paraffin-embedded diagnostic lymph node biopsy samples from 15 HIV-positive patients were donated.
Their racial identification could not be done because over a half of them were consultation cases. All the patients were confirmed to be HIV-positive, but were apparently free from AIDS-NHL. In addition, 20 lymph nodes obtained from HIV-negative patients were used as controls.
Methods
First, we carried out ISH to identify the EBER-1. Then, to clarify the expression of LMP-l on EBER-1-bearing cells, immunostaining was overlapped on the same slides to which the ISH was carried out. To further investigate the size of EBER-l-bearing cells, we measured the cell area using a video analysis program (Olympus-Avio SP500, Olympus,Tokyo).
In situ hybridization
The probe used to detect EBER-l by ISH was prepared using an OIigo 1000 DNA Synthesizer (Beckman, CA, USA), and was a 30-base oligonucleotide (5'-AGA CAC CGT CCT- CAC CAC CCG GGA CTT GTA-3').8 The probe was labeled with digoxygenin according to the digoxigenin 3'-end-labeling method using a DIG oligonucleotide 3'-end-labeling kit (Boehringer Mannheim, Mannheim, Germany).9 The ISH procedure was as follows: 4μm thick sections on slides were deparaffinized and rehydrated by serial immersion in xylene and ethanol. In order to reduce non-specific reaction, the sections were first rinsed in 0.2N HCI. The sections were then pretreated with 80μg/mL pronase (Wako Pure Chemical Co., Osaka, Japan) to increase their permeability to the probe, and dehydrated in ethanoI. The specimens were acetylated in 0.l moI/L triethanolamine at pH 8.0 for 10min with 0.25% acetic anhydride, and incubated with hybridization buffer containing the EBER-1 probe. The samples were then blocked by Buffer 2 of the DNA tailing kit (Boehringer Mannheim) and washed. For color development, alkaline phosphatase-labeled anti-digoxigenin antibody (Boehringer Mannheim) was introduced. Nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP; Promega, Madison, WI, USA) was used as the chromogen. Diethyl-pyrocarbonate (DEPC; 0.1%) was used throughout the procedure to avoid RNase reaction.The number of positively stained cells per visual field at ×400 magnification was calculated as the average of 10 fields, and the results were expressed semiquantitatively as+++, 10 cells or over; ++, less than 10 cells and over 1 cell; and +, less than 1 cell per field.
Immunohistochemical staining
The avidin-biotin complex (ABC) method was used for immunohistological analysis.10 As the first-step antibody for detection of LMP-1 , CSI-4 (antil-MP-l murine monoclonal antibody; DAKO Japan, Kyoto, Japan) was used. This antibody was formerly shown to be applicable to paraffin-embedded specimens. Biotinylated anti-mouse immunoglobulin antibody (DAKO Japan) was used as the second-step antibody and peroxidase-conjugated streptavidin (DAKO Japan) as the third-step reagent. An AEC Kit (Nichirei, Tokyo, Japan), using aminoethyl carbasol as chromogen, was used for color development of peroxidase.
Double labeling
Some of the slides used for ISH were also double-immunostained for EBER-l and IMP-1. Antibodies used for the immunostaining were those listed previously. The slides were first stained for EBER-1 , rinsed in phosphate-buffered saline, and then immunostained for LMP-l , since we could not get good results by following the opposite sequence (i.e. immunostaining first and ISH thereafter).
Video analysis
An Olympus-Avio SP500 video analyzer and a matching two-dimensional video analysis program were used to measure the size of EBER-l-bearing cells. This equipment can separate a specimen into 256 phases by light absorption, and thus can determine the staining intensity of the cell. Its operating procedures include binary processing, image contrast enhancement, binary image measurement, circle edge detection and measurement, compilation of a distribution graph, compilation of a correlation graph and pseudo-color processing.
Histological classification of lymph nodes
The lymph node specimens studied were classified histologically into four subtypes according to the criteria of Grundmann: (i) irregular follicular hyperplasia (IFH); (ii) at the beginning of follicular destruction (BFD); (iii) progressive follicular destruction (PFD); and (iv) follicular involution (FI).11
RESULTS
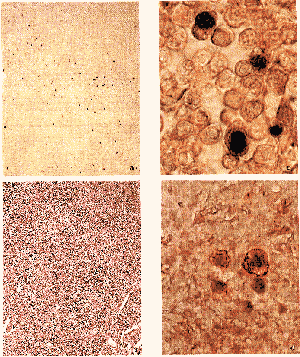
Figure 1
(a) Low-power field of a lymph node obtained from a HIV carrier (case 1) demonstrating Epstein-Barr virus-encoded RNA-1(EBER-1) by in situ hybridization. (b) Hematoxylin-eosin staining of the same section. (c) High-power field of a. (d) Double-staining of latent menbrane protein-1(red)and EBER-1(black) in case 1.
EBER-1-bearing cells were identified in nine of the 15 lymph node specimens studied. ISH showed a positive reaction for EBER-l exclusively in the nuclei (Fig. 1a,c). When the numbers of EBER-1-positive cells were counted, one case was classified as +++, two cases were classified as ++, and six as (+). Six cases were classified as (-)(Table 1). Nine additional cases were studied by ISH, but excluded from the results because of an apparent non-specific reaction in endothelial cells or fibroblasts. In contrast, no EBER-1-positive cells were identified in any of the 20 control lymph nodes obtained from. HIV negative patients.
The histological subtypes of these 15 specimens were IFH in six cases, BFD in four cases, PFD in two cases and FI in three cases. Among these, the nine cases that were EBER-1 -positive were l/6 cases showing IFH, 3/4 showing BFD, 2/2 showing PFD and 3/3 showing Fl (Table 1).
TopologicaIIy, EBER-1-bearing cells were detected mostly in two areas, the perifollicular area and the lymph follicule, white much fewer EBER-1-positive cells were observed in the T-zones (Fig.1 a,b). Meanwhile, the basic structure was totally effaced in lymph nodes where EBER-l-positive cells were most numerous (Fig. 1c).
With regard to cell size, a proportion of EBER-1-positive B cells were small or medium-sized, while some were large or even giant. Two-dimensional video analysis showed that the smallest area of each EBER-1-positive cell was 15μm2, in contrast to 9.20±2μm2 for EBER-1-negative cells. The largest EBER-1-bearing cell had an area of 75μm2. On double staining with EBER-1 and LMP-1, LMP-1-expressing cells were shown to be much larger (Fig. 1d): EBER-1(+) LMP-1 (+), 62.83±6μm2; EBER-1 (+) LMP-1 (-), 29.05±7μm2; and EBER-l(-) LMP-1(-), 9.20±2μm2 (Table 2), indicating that the LMP-1-expressing cells had a much larger cell area. It is worth adding that no significant difference was noted in the cell sizes of each group among different cases (Table 2).
DISCUSSION
The present study clearly showed that (i) the number of EBV-infected cells is increased in lymph nodes of HIV-infected individuals; (ii) some, if not all of such EBV-infected cells express LMP-1; and (iii) some of the EBV-infected and LMP-1-expressing cells are in an activated state, as observed by the enlargement of their cytoplasm. The identification of EBV-infected cells in lymphoid organs of HIV carriers has been reported by several groups, of which at least three have demonstrated EBV DNA using DNA-ISH.12-14 However, the results of these three studies were somewhat contradictory. Shibata et al.12 demonstrated EBV DNA in as many as 10/16 lymph nodes that were in the persistent generalized lymphadenopathy (PGL) stage of the WHO system, while Uccini et al.13 (0/30) and Chappuis et al.14 (0/15) could not demonstrate EBV DNA-bearing cells using the same technique.
Table 1
The ,number of EBER-1 and LMP-1-expressing cells in lymph nodes of HIV-infected individuals
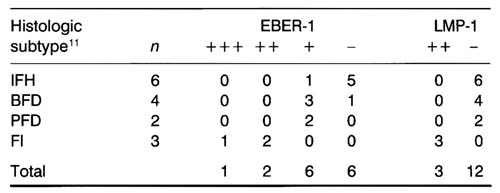
+++, over 10 positive cells*/1 high power field; ++, less than 10 positive cells*/1 high power field; +, less than 1 positive ceIIs*/1 high power field; -,positive cells are not observed; (*, mean value of 10 high power field). .IFH, irregular follicular hyperplasia; BFD, beginning follicular destruction; PFD, progressive follicular destruction; FI, foIIicular involution; EBER-1 , Epstein-Barrvirus encoded RNA-1 ; LMP-1 , latent membrane protein 1.
Table 2
Area of E.BER-l/LMP-1-expressing cells in lymph nodes of HIV carriers
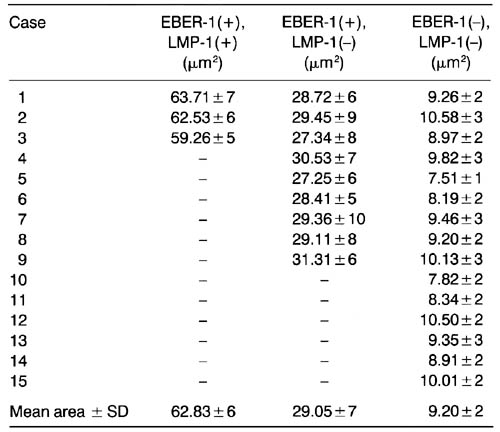
Ten cells of each case were measured using an Olympus-Avio SP500 video analyzer. For each case area/cell ± SD is given. EBER-1 , EpstleinBarr virus-encoded RNA-1 ; LMP-1, latent membrane protein 1.
Actually, demonstration of EBV DNA is far more difficult than EBV RNA (EBER-1) with the use of ISH on EBV-transformed cells. AIso, a very small number of cells could be stained by DNA ISH of EBV-infected biopsy/autopsy specimens, whatever their form, and thus it can be speculated that the data of Shibata et al. were over-evaluated. The detection of EBER by ISH, on the other hand, is currently regarded as one of the most sensitive methods for demonstrating EBV-infected cells.
To our knowledge, however, an attempt to demonstrate EBER-1 on such HIV-infected non-neoplastic lymph nodes has been reported by only one group.15 This work, done by Borisch et al. was a small-scale study in which they demonstrated EBER-positive cells in only one out of five non-neoplastic lymph nodes. No further information was available about the nature of the cells examined or the patient's status. Thus, the present study appears to be the first systematic analysis of EBER-expressing cells in non-neoplastic lymph nodes from HIV carriers.
We demonstrated that most of the lymph nodes containing these EBER-1-bearing cells were at stage BFD, PFD or FI, while a large proportion of IFH cases were EBER-1-negative. Hence, it is obvious that EBV-positive cells are much more common in HIV carriers, especially those who are at an advanced stage.
We also found that LMP-1 was more easily identified when the patient was at the most advanced stage of HN infection. In fact, LMP-1-positive cells were found exclusively in stage FI.
Furthermore, the cells expressing both EBER-1 and LMP-1 were revealed to be far larger, implying that they were in an activated/transformed state. It is well known that peripheral B cells are transformed easily by in vitro infection with EBV, and such EBV-transformed B cells, termed lymphoblastoid cell line (LCL) have a large blastoid morphology, expressing LMP-1 on the cell membrane and EBER-1 in the nucleus. Thus, it is highly probable that the EBER-1-positive and LMP-1-positive large cells observed in the present study correspond to the LCL. Also, these data clearly suggest that EBV-transformed and IMP-1-expressing B-cell transformants occasionally exist in lymphoid tissues of HIV-infected patients who are at a late disease stage. It is highly probable that these transformed cells are the precursors of B-NHL occurring in immunocompromised patients. One problem that needs to be addressed if this hypothesis is to be accepted, is that most of the B-NHL in immunocompromised hosts occur in extranodal organs, and only a very small number in lymph nodes. The discrepancy between the extranodal occurrence and the presence of EBV-transformed cells in lymphoid organs requires further clarification.It might be important to clarify the immunophenotypic characters of EBER-1 + and/or EBER-1 + LMP-1 + cells observed in the present study. We tried to do this intensively with the introduction of double or triple staining. The results, however, were ambiguous, and we could not obtain definitive results. Further studies based on un fixed frozen sections are being conducted.
Finally, the present study did not include the follow-up data and the racial identification of each case. This is because over half of them were consultation cases from different hospitals and, thus, it was practically impossible to get the additional full data on these cases.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Education, Science and Culture. We are grateful to Professor Marshal Kadin (Beth Israel Hospital, Boston, USA), Dr GIauco Frizzera (Armed Forces Institute of Pathology, Washington, USA), Dr Shiro lmahori (Erie County Hospital, Buffalo, USA), and Dr Tomo Wakabayashi (Institute of Medical Science Hospital, University of Tokyo, Tokyo, Japan) for kindly donating the specimens used in this study.
REFERENCES
- Kaplan LD, Abrams DI, Feigal E et al. AIDS-associated non-Hodgkin's lymphoma in San Francisco. JAMA 1989; 261:719-724.
- Herndier BG, Kaplan LD, McGrath MS. Pathogenesis of AIDS lymphomas.AIDS 1994; 8: 1025-1049.
- Pallesen G, Hamilton-Dutoit SJ, Rowe M et al. Expression of Epstein-Barr virus replicative proteins in AIDS-related non-Hodgkin's lymphoma cells. J. Pathol. l991 ; 165: 289-299.
- Subar M, Neri A, Inghirami G, Knowles DM, Dalla-Favera R. Frequent c-myc oncogene activation and infrequent presence of Epstein-Barr virus genome in AIDS-associated lymphoma. Blood 1988; 72: 667-671.
- Hamilton-Dutoit SJ, Pallesen G, Franzmann MB et al. AIDS-related lymphoma.Am. J. Pathol. 1991 ; 138: 149-163.
- Hamilton-Dutoit SJ, Raphael M, Audouin J etal. ln situ demonstration of Epstein-Barr virus small RNAs (EBER1) in acquired immunodeficiency syndrome-related lymphomas: Correlation with tumor morphology and primary site. Blood 1993; 82:619-624.
- Robin F, Lars R, Johng SR, George K. Morphological transformation of human keratinocytes expressing the IMP gene of Epstein-Barr virus. Nature 1990; 345: 447-449.
- Chang KL, Chen YY, Shibata D, Weiss LM. Description of an in situ hybridization methodology for detection of Epstein-Barr virus RNA in paraffin-embedded tissues, with a survey of normal and neoplastic tissues. Diagn. Mol. Pathol. 1992; 4: 246-255.
- Nagasato H, Tokunaga M, Oyamada M et al. Certification method of Epstein-Barr virus (EBV) in pathologic specimens: In situ hybridization in pathologic laboratory. PathoI. Clin. Med. 1992; 10: 951-955.
- Mori S. Immunohistology as a diagnostic tool in routine laboratory medicine. Mod. Media 1986; 32: 10-l9.
- Grundmann E. Immune reactions in the lymph node in cancer and AIDS. Tokyo J. Med. Sci. 1990; 97: 68-69.
- Shibata D, Weiss LM, Nathwani BN, Brynes RK, Levine AM. Epstein-Barr virus in benign lymph node biopsies from individuals infected with the human immunodeficiency virus is associated with concurrent or subsequent development of non-Hodgkin's lymphoma. BIood 1991 ; 77: 1527-l533.
- Uccini S, Monardo F, Vitolo D et al. Human immunodeficiency virus (HIV) and Epstein-Barr virus (EBV) antigens and genome in lymph nodes of HIV-positive patients affected by persistent generalized lymphadenopathy (PGL). Am. J. Clin. Pathol. 1989; 92: 729-735.
- Chappuis BB, MuIIer H, Stutte J, Hey MM, Hubner K, Muller Hermelink HK. Identification of EBV-DNA in lymph nodes from patients with lymphadenopathy and lymphomas associated with AIDS. Virchows Arch. B. Cell Pathol. 1990; 58: 199-205.
- Borisch B, Finke J, Hennig I et al. Distribution and localization of Epstein-Barr virus subtypes A and B in AIDS-related lymphomas and lymphatic tissue of HIV-positive patients. J. Pathol. 1992: 168: 229-236.